The term autoxidation designates a complex set of reactions which result in the fixation of oxygen by lipids and shows complex autocatalytic behaviour and involves a number of interrelated reactions of intermediates. The observation that saturated lipids only autoxidise very slowly means that this reaction occurs involving the isolenic unsaturated double bonds present. Autoxidation of food is usually modelled by that of a simpler system in which either a triacylglycerol with three equal acyl groups or a simple ester are used as models in the presence of oxygen under controlled experimental conditions, and the products obtained at various stages and rates of interconversions observed are studied.
These studies have shown that many variables can affect autoxidation rate. Thus fatty acid composition, light, transition metal ions, oxygen pressure, presence of antioxidants, prooxidants, temperature, moisture content and distribuition were shown to affect the rate of the reaction.
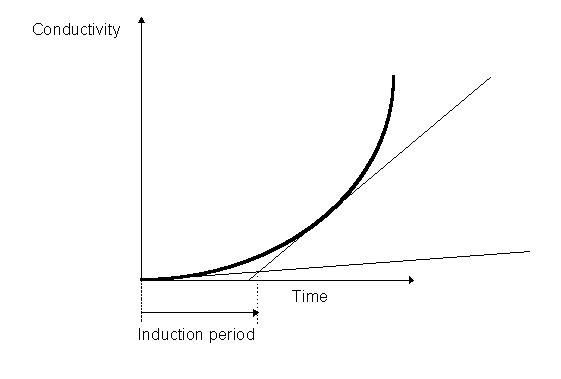
It may be stated that this reaction, though still not completely defined, seems to occur at an increasing rate, after an initial stage called an induction period.
Autoxidation leads to products with higher oxygen content. Some of these are more volatile and their low sensory threshold conveys an early warning of their presence. Others are polar compounds such as acids and they will confer to the oxidising food a higher conductivity. Still another range of products are polymeric.
The polar compounds form the basis of detection in a method for measuring oxidative stability of oils. In this method, which is only one of a huge series of methods - confirming the idea that autoxidation is complicated - a sample of oil is subject to high temperature (say 100ºC) in a vessel which operates under moderately high oxygen pressure, and conductivity is measured by way of electrodes. The induction period before quick rise in conductivity measures the oxidative stability of the oil in this so called "Rancimat" method.

Induction period and realtive rate of oxidation for fatty acids at moderate temperatures is strongly dependent on unsaturation. An approximate idea of relative reactivities and induction periods (which must be considered as a mean value because factors such as light intensity, photosensitizers and antioxidants would alter these relative values for oils), is given below:
| Fatty acid | Number of allyl groups | Induction period (h) | Relative rate of oxidation |
| Stearic | 0 | - | 1 |
| Oleic | 1 | 82 | 100 |
| Linoleic | 2 | 19 | 1200 |
| Linolenic | 3 | 1.34 | 2500 |
Both the induction period and the rise in the reaction rate in the series oleic, linoleic and linolenic acid can be explained assuming that oxidation proceeds by a sequential free radical chain-reaction mechanism. Relatively stable radicals that can abstract H-atoms from the allylic methylene groups in olefinic compounds are formed. The oxidation process is therefore a radical-induced chain reaction which may be divided into the classical steps of initiation, propagation, branching (and this one explains the autocatalysis) and termination. Initiation results in the formation of free radicals P· by a variety of processes, and this is able to generate an alkyl radical E· .
| ........ ® P· | INITIATION | [1] |
| P· + EH ® PH + E· | INITIATION | [2] |
| E· + O· ® EOO· | PROPAGATION | [3] |
| EOO· + EH ® EOOH + E· | PROPAGATION | [4] |
| EO· + EH ® EOH + E· | PROPAGATION | [5] |
| EOOH ® EO· + ·OH | BRANCHING | [6] |
| 2EOOH ® EOO· + EO· + H2O | BRANCHING | [7] |
| 2E· ® products | TERMINATION | [8] |
| 2EOO· ® products | TERMINATION | [9] |
| E· + EOO· ® products | TERMINATION | [10] |
The reaction rate constants for the different steps of this radical chain reaction markedly differ in magnitude, especialy because of the stability of the peroxy free radicals (EOO·.) which reacts so slowly that it conditions and limits the overall oxidation rate. Thus formation of monohydroperoxide molecules (EOOH) which is achieved by abstraction of an H-atom from a fatty acid molecule, is the slow process in the formation of radicals.
Branching, shown in reactions 6 and 7 above, is responsible for the autocatalytic effect, in as much as it increases the number of reacting chains, and conversely, termination diminishes the number of reacting chains. it is obvious that these later reactions only happen when the number or radical species is high, for they depend upon the fact that two of these meet before either finds another adequate substrate.
Branching as shown in reaction 7, though exothermal in contrast to reaction 6, only becomes significant when hydroperoxide concentration is likewise high, a condition which normaly is fulfilled only long after the food item is no longer considered edible. Reaction 6 will be catalysed by transition metals and their complexes, which in this way act as prooxidants since the radicals produced in these reactions can start chains anew.
Each new radical can start a chain responsible for many molecules of hydroperoxide, before a termination reaction stops it, and if there is air enough it will keep adding to the allylic positions. In a high oxygen atmosphere, such as air, termination by reaction [9] of two peroxy radicals is more probable. The resulting species has a labile tetraoxygen group which decomposes rapidly yielding singlet oxygen and two new chains, and is therefore not a pro bone termination recation at all, for singlet oxygen has oxidising capabilities which far exceed those of triplet oxygen.
Termination reactions depicted as [8] and [10] above play a role when the oxygen level is low, as may happen in the inner portion of a fatty food, or under high temperature conditions..
The whole scheme presented so far is an accurate description of phenomena at the early stages of autoxidation. Nevertheless more must be said about initiation, and also about the fate of peroxides formed and the importance of the role of their decomposition or further oxidation products with the initial products of autoxidation.
Initiation of radical chain reactions