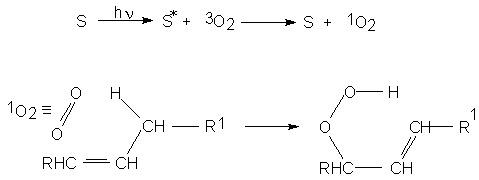
The knowledge of the reactions which predominate during the induction period is important because it may help find out how to make it last longer, and thus improve the resistance of oils to oxidative degradation.
Two different types of reactions have been recognized as important in this process.
The first of these has to do with the initiation reactions which are responsible for by-passing the energy barrier required for oxidation of allylic groups and includes photosensitised oxidation (photooxidation) and lipid oxidation by lipoxygenase catalysis.
Both of these provide initial hydroperoxides, which are further converted into radicals by reactions of a second type.
Heavy metal ions and heme groups may be involved in reactions of this second type.
Enzyme catalysed reactions generating the superoxide radical anion can be placed in between theses two types of reactions, for they need also H2O2 to be able to initiate radical reactions.
A.Photooxidation
In order to understand photooxidation, or light promoted oxidation, and to differentiate it from autoxidation, it should be understood that the oxygen ground state is a triplet, but that an excited singlet is available with an energy only 92 kJ/mole above that of the ground state.
Whereas triplet ground state oxygen tends to react as a diradical, using its semioccupied orbitals for the purpose of building new bonds and preferring other radicals as substrates, the excited singlet may use wholy occupied and/or empty orbitals for the same purpose, hence behaving as an nucleophile/electrophile and participating in electrocyclic reactions and reacting with other molecular entities.Hence, in the reaction with oleic acid, the singlet state oxygen attacks the 9-10 double bond producing an equimolar mixture of the 9- and 10- hydroperoxides (R and S).
Light can trigger lipid oxidation in two different ways, both mediated by small amounts of compounds called sensitisers.
The first type of photosensitiser, Type I sensitisers (S), once activated by light (S*), reacts directly with a substrate, generating radicals which are the initiators of the oxidation process.
The other sensitisers are those which activate the ground state of oxygen to the first singlet state, and they are called Type II photosensitisers.
Photooxidation processes involving both types of sensitisers occur simultaneously, and both the structure and availability of the sensitisers present as well as the concentration and structure of the substrate available for oxidation are involved in determinig which one will prevail.
As the hydroperoxide isomer distribution resulting from oxidation with singlet and triplet oxygen differ, it is possible to determine the relative importance of these two mechanisms by product analysis.
Thus, one can distinguish Type I from Type II photooxidation, and in this way, prove that sensitisers such as chlorophylls, pheophytins, and riboflavin, commonly present in food items will catalyse the Type II oxidation of compounds containing unsaturated acyls.
The formation of hydroperoxides exclusively at former double bond (sp2) carbon atoms of unsaturated fatty acids may be seen in the table above, the actual formulas of the four (S,R) racemates formed evidencing also the preferential cis-trans isomerisation which occurs when linoleic acid is oxidised by singlet oxygen. It may be seen that, in addition to the two hydroperoxides with conjugated diene system, two others are obtained with isolated double bonds.

Reaction of singlet oxygen (1O2) with double bonds is inhibited by carotenoids present, for these compete for the 1O2 depriving it of its excess energy and making it revert to the ground state triplet, whereas the carotenoid itself becomes a triplet but dissipates excess energy thermally and reverts to a singlet. This quenching effect is very fast (k = 3 x 1010 mole-1s-1), therefore carotenoids are particularly suitable for protecting fat (oil)-containing food from Type II photooxidation. Their role as auxiliary pigments in photosynthesis means they ubiquitously accompany chlorophyll, whose prooxidant effect they effectively tend to nullify.
B.Heavy metal ions
Fats, oils and foods always contain traces of heavy metals, the complete removal of which in a refining step is not industrially performable. These ions, primarily Fe, Cu and Co, may originate from impurities accompanying the plant material, packaging of oilseeds or oil, or from processing equipment. These ions are responsible for initiation reactions of the second type, by catalysing the decomposition of hydroperoxides into radicals which initiate new radical chains in the oxidation process.
Vegetable oils of the linoleic acid type, such as sunflower and corn germ oil, which are easily oxidisable, should contain less than 0.03 ppm Fe or 0.01 ppm Cu to ensure acceptable stability. This may be as high as 5 ppm for both Cu and Fe in animal fats with a high content of oleic and/or stearic acid.
The presence of a hydroperoxide group is a prerequisite for metal ion activity as an oxidation catalyst leading to hydroperoxide decomposition and new radical chain initiationl:
The first of these reactions is much faster than the second, hence the alkoxide radicals more important initiators than the peroxide. Water phase antioxidants such as ascorbic acid can prove to have here a prooxidant effect for they tend to reduce oxidised metal ions to their lower oxidations states, hence permitting more of the first reaction above to take place. In this case there is also a pH effect, but normal values for food are near-optimal for metal ion redox catalysis of peroxide decomposition.
In any case, it is important to exclude hydroperoxides or diminish their concentration.
Last but not least water activity will condition autoxidation. Both very low and very high water activity values seem to promote food oxidation, the minima occuring at intermediate aw values of 0.25-0.3.
At high water activity prooxidant mobility (including enzymes) is the probable reason for this, and at very low water activity, increased oxygen mobility permited through positions left vacant by removed water molecules.
C.Heme compounds
Proteins exhibiting heme-like prosthetic groups are ubiquitous and an indispensable part of the electron transport chain. They can chelate peroxides cleaving them into an alkoxy and a hidroxy radical and then releasing them, especially after having been denatured in order to expose the heme groups. this effect is not dependent on pH or added ascorbic acid as it does not involve an alteration of the oxidation state of the metal ion.
Denaturation of lipoxygenase which is the main enzymatic culprit for oxidation of vegetable foods must be done with care not to denature catalase or peroxidase too strongly, as this may expose their heme groups which will become oxidation catalysts.
D.Activated oxygen from enzymatic reactions
As part of the biochemical mechanisms entailing the use of oxygen, an enzyme cascade is involved. These reactions entail the formation of a series of progressively more reduced species, in an aqueouis environment. Thus if oxygen, or rather dioxygen is the most oxidised form, and water is the reduced form, these intermediate forms are, in first place, the superoxide anion, which is the result of a one electron reduction of dioxygen, and behaves as a nucleofile, and its protonated form the hydroperoxide radical which only is formed under conditions rather more acidic than physiological ones.
Superoxide generated by the flavin enzymes will slowly dismutate into dioxygen and hydrogen peroxide, a reaction which can also be catalysed by superoxide dismutase. Hydrogen peroxide can suffer photochemical or chemical decomposition yielding the extremely hard and reactive hydroxyl radical, unless it is first reduced to water by catalase. A particularly efficient way of generating the hydroxyl radical is the so-called Fenton reaction, whereby a transition metal ion complex catalyses the oxidation of superoxide to dioxygen while generating the hydroxyl radical from hydrogen peroxide:
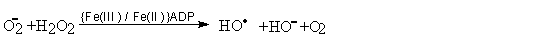
Fenton reaction
The hydroxyl radical formed will initiate autoxidation.
Reactions of primary oxidation products
The hydroperoxides formed in the primary double bond oxidation process of unsaturated acids and lipids are not stable, but they also do not possess any noticeable sensory properties. They will nevertheless promptly react under normal autoxidation conditions yielding a series of compounds which have extremely low sensory detection thresholds. These mainly comprise carbonyl compounds and a few hydrocarbons, but substituted furanes and alcohols are also formed.
Main volatile carbonyl compounds from unsaturated fatty acids after uptake of ½ mole oxygen, (in ppm).
| Oleic acid | Linoleic acid | Linolenic acid | |||
| Heptanal | 50 | Pentanal | 55 | Propanal | major |
| Octanal | 320 | Hexanal | 5100 | 1-Penten-3-one | 30 |
| Nonanal | 370 | Heptanal | 50 | 2tr-Butenal | 10 |
| Decanal | 80 | 2tr-Heptenal | 450 | 2tr-Pentenal | 35 |
| 2tr-Decenal | 70 | Octanal | 45 | 2c-Pentenal | 45 |
| 2tr-Undecenal | 85 | 1-Octen-3-one | 2 | 2tr-Hexenal | 10 |
| 2c-Octenal | 990 | 3tr-Hexenal | 15 | ||
| 2tr-Octenal | 420 | 3c-Hexenal | 90 | ||
| 3c-Nonenal | 30 | 2tr-Heptenal | 5 | ||
| 3tr-Nonenal | 30 | 2tr,4c-Heptadienal | 320 | ||
| 2tr-Nonenal | 30 | 2tr,4tr-Heptadienal | 70 | ||
The odour threshold values of these compounds are low or even very low, and can be lower in a polar medium than in a lipid phase where their solubility is high.
The autoxidation of a-linolenic acid produces especially strong odours, and it is therefore extremely easy to spot early.
Of these one should stress 3-cis-hexenal and 2-trans,6-cis-nonadienal. Other carbonyl compounds with similar aromatic strengh and low threshold will be released from animal fats contributing to a characteristic "warmed-up" flavour, or when processing and especially if re-processing vegetable oils.
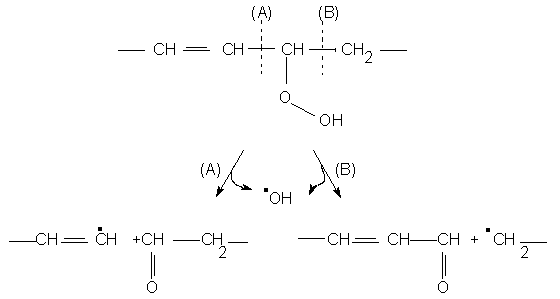
The b-scission of hydroperoxides catalysed by heavy metal ions or heme compounds is the main pathway explaining the formation of these carbonyl compounds. Of the two different mechanistic possibilities (A) and (B), shown in the figure above, the second one is energetically preferred, the transition state leading to it presumably lower in energy than that involved in (A), as might have been hinted at by the fact that a conjugated carbonyl compound is obtained through (B). Though some of the structures of products obtained may be rationalised in this way, as is the case for 2,4-decadienal and pentane can be explained in this way, this is not the case for hexanal, whose preferential formation in aqueous phases may point to an ionic mechanism.
The further oxidation reactions of monohydroperoxides and carbonyl compounds are among the possibilities of explaining some of the products obtained and which cannot be obtained through (B).
Sensory properties of aliphatic aldehydes and vinyl ketones
| Compound | Odour Description | Odour Threshold value (ppb) in | |||||||||
| sharp | oily fatty | tallowy | frying odour | green leafy | cucumber like | fishy | orange peel like | metallic | paraffin oil | water | |
| Aldehydes | |||||||||||
| 5:0 | + | 100 | 10 | ||||||||
| 6:0 | + | + | 150 | 4.5 | |||||||
| 7:0 | + | 45 | 30 | ||||||||
| 8:0 | + | 50 | 40 | ||||||||
| 9:0 | + | 250 | 40 | ||||||||
| 10:0 | + | 900 | 5 | ||||||||
| 5:1 (2tr) | + | + | 700 | - | |||||||
| 6:1 (2tr) | + | + | 1500 | 17 | |||||||
| 6:1 (3c) | + | 100 | 0.3 | ||||||||
| 7:1 (2tr) | + | 14000 | 50 | ||||||||
| 8:1 (2tr) | + | 7000 | 4 | ||||||||
| 9:1 (2tr) | + | + | 3500 | 0.08 | |||||||
| 7:2 (2tr,4c) | + | + | 50 | - | |||||||
| 7:2 (2tr,4tr) | + | 10000 | - | ||||||||
| 9:2 (2tr,4tr) | + | 460 | 90 | ||||||||
| 9:2 (2tr,6c) | + | 2 | 0.05 | ||||||||
| 9:2 (2tr,6tr) | + | + | 240 | 1 | |||||||
| 10:2 (2tr,4c) | + | 20 | - | ||||||||
| 10:2 (2tr,4tr) | + | 200 | 0.07 | ||||||||
| 10:3 (2tr,4c,7c) | + | - | - | ||||||||
| Vinyl ketones | |||||||||||
| 1-Penten-3-one | + | 3 | 1 | ||||||||
| 1-Octen-3-one | + | 0.1 | 0.1 | ||||||||
| 1,cis-5-Octa- dien-3-one | + | + | 0.1 | 0.001 | |||||||
That tandem oxidation processes are ocurring is supported by the fact that 2-alkenals and 2,4-alkadienals suffer oxidation at faster rate than the parent unsaturated fatty acids. The autoxidation of 2,4-decadienal yields hexanal and other volatiles which fit into the pattern obtained from linoleic acid oxidation. Saturated aldehydes will be more stable and eventualy predominate in the mixture.
Other compounds such as pentanal need as precursors the oxygen heterocycles which may result from peroxide intramolecular rearrangements.
The autoxidation of fatty acids with three or more double bonds invariably leads to high yields of malondialdehyde. This compound may serve as a protein denaturing reagent by crosslinking with two amino groups. Malondialdehyde may result from alinoleic acid, and its presence is used as an indicator for fat or oil oxidation (TBA -thiobarbituric acid test) where a colour producing reaction is used as an indicator.
Several furan derivatives occur among the autoxidation products of linoleic and linolenic acids. These derivatives may be involved in the development of an off flavour specific to soyabean oil, removable in the refinery but which may come back if oxidation is allowed to start again, in a phenomenon termed flavour reversion.
The main hydrocarbon constituents of the volatile hydrocarbon fraction, ethane and pentane can easily and quantitatively be detected by headspace chromatography and are used as indicators of lipid autoxidation.